Multiphoton / 2-Photon, Olympus
The Multiphoton / 2-Photon imaging system from Olympus allows outstanding fluorescence imaging down into deep regions of the specimens.
Applications in brief
Our multiphoton imaging system is purpose-built for deep imaging in biological tissue, aimed at revealing both detail and dynamics. Molecules can be visualized deep inside the specimen enabeling 3D imaging of tissue slices, organoids, whole organs, embryos, or even whole animals.
Efficient delivery and detection of photons in scattering media enable high signal-to-noise ratio acquisition, high sensitivity, and high-speed imaging to capture fast in vivo responses.
Which samples may I use?
Selected samples, but not limited to:
- organoids
- whole organs
- embryos and whole animals
Training and use
Filter configuration
Ion deposition filter sets included to efficiently detect fluorescence light, e.g. CFP/YFP, GFP, Calcium indicators, etc.
Filter cubes can be exchanged for detection of different dye combinations. Please refer to this FILE to configure your setup. If you need additional filter cubes we will help configuring a cube for you to purchase.
MPE laser tuning
The MPE system is equipped with a femtosecond laser that is tunable from 690nm - 1040nm.
When you need to excite multiple fluorophores, my best guide is to start out with 800nm and detect the signal in all channels. If you are missing signals, please go to this site and search for the fluorophore (with the extension “2P”) and tune the laser towards the specified wavelength.
http://www.spectra.arizona.edu/
Laser intensity may drop at extreme wavelengths and it is usually better to use e.g. 1020 than 1040nm
Specifications
Microscope
- Nosepiece focusing, fixed stage upright BX63L microscope
- Transmitted light condenser for Olympus water immersion objectives
XY stage
- Motorized XY stage (Prior Z-Deck for high-precision repeatability), joystick controlled
- Linear encoders for increased accuracy
- XY stage control software module to perform advanced experiment procedures, implemented in FluoView application software
- Automatic complex multi-area scanning in combination with FVMPE-RS “MATL” software module to program complex acquisition protocols (see FVMPE-RS software below
Objective
- Water immersion objective with isolated tip
- TruResolution objective designed for multiphoton excitation up to 1300 nm
XLPLN25xWMP2: NA 1.05 - WD 2.00 mm
high performance objective for multiphoton excitation (high IR-transmission from 400 to 1600 nm)
The objective maximize resolution and contrast for 3D imaging deep within thick specimens. The objective is equipped with a motorized correction collar (adapting to refractive index mismatch) that can automatically and dynamically compensate for spherical aberration while maintaining focus position.
Ocular view
Find your specimen with transmitted light or wide-field fluorescence:
- Fluorescence mirror unit for GFP
- XL Fluorescence mirror unit (Wideband Green excitation), Green excitation: e.g. Rhodamine/TRITC, Cy3, Texas Red, Alexa Red, RFP
Multiphoton excitation and non-confocal detection,
incl. 4 non-confocal/non-descanned detectors + one standard external transmitted light detector, IR laser introduction with ultra-fast AOM intensity control.
This section includes all the FluoView specific adaptations to the microscope frame to setup a complete FluoView multiphoton excitation laser scanning microscope system according to the provided system requirements:
Hybrid scan unit for multiphoton excitation imaging
· The hybrid scan unit is designed for highly effective and highest speed IR laser scanning. High IR transmission is achieved with anti-corrosive silver coated galvanometric scanning mirrors.
· Fibre coupled conventional fluorescence illumination via the scan unit and software controlled motorized switch on/off.
· Scan unit is equipped with a set of high-performance galvanometer point scanners and a set of high speed resonant galvonometer scanners. Switching between standard point and resonant scanning can be done conveniently via software and takes appox. 3 seconds.
Non-descanned detector unit with 4 detection channels in reflection
· Non-descanned reflection detector module with 4 channels implemented close to the specimen position to observe fluorescence in reflection mode.
2 high-performance multi-alkali PMT detectors
2 GaAsP PMT detectors
Ion deposition filter sets included to efficiently detect fluorescence light, e.g. CFP/YFP, GFP, Calcium indicators, etc. Filter cubes can be exchanged for detection of different dye combinations (see next section " Filter configuration").
Fully automated beam introduction system for pulsed IR laser including AOM laser control
- IR laser introduction and beam auto alignment system for pulsed IR laser introduction into hybrid scanner unit of FVMPE-RS.
- Ultra-fast AOM (acousto optical modulator) laser on/off control and attenuation with IR laser introduction light path, flyback beam blanking and shuttering speed below 10 microseconds.
- Motorized Keplerian beam expander with IR laser introduction light path to achieve perfect excitation efficiency and highly resolved MPE images
- Deep Focus Mode to boost excitation efficiency while imaging in deep sample regions
- Quadralign 4-Axis Auto-Alignment: This auto alignment mechanism tunes the optical axes of the IR laser by 2 horizontal and 2 angular axes. Single-click auto-adjustment on demand is also enabled for laser xy beam position as well as incident laser angle — a common cause of pixel shift
- Whole optical system is designed for low dispersion and thus for several hundred micron deep imaging of specimen.
IR laser - for multiphoton laser imaging and manipulation / stimulation
- MaiTai DeepSee Olympus version specifically tailored for FVMPE-RS system
- Automatic optical adjustment of negative chirp when changing IR laser wavelength
- Mai Tai laser equipped with automated dispersion compensation, delivering high peak power to the sample thus maximizing the fluorescence signal.
Peak Power | Pulse Width | Tuning Range | Average Power | |
MaiTai HP DS-OL | >266 kW* | <100 fs* | 690–1040 nm* | >2.1 W* |
Z-Axis control with the BX63L
Stepper motor for control of Z-axis position is integrated in BX63L microscope frame, minimum step size 10 nm (the nosepiece movement is controlled in z-direction whereas microscope stage is fixed).
Note:
FluoView software allows specific selection of z-focus working range, maximum working range 20 mm.
FluoView Software
- Scan speed
- standard one-way scan 5Hz - 500 Hz
=> e.g. 1 frame per second at 500Hz with 512 x 512 pixels - resonant scan at 8000 Hz
=> 30 frames per second at 512 x 512 pixels
=> 438 frames per second at 512 x 32 pixels
- standard one-way scan 5Hz - 500 Hz
Note: Resonant scan is performed at a field number of 18 allowing a big field of view even at highest speed
- Optical zoom of the scanning system
- Standard galvano scanner: 1x to 50x in 0.1x steps
- Resonant galvano scanner: 1x to 8x in 0.1x steps
Standard image pixel formats
Galvo scanning
ranging from 64x64 to 4096x4096 pixels. 12 bit per channel acquisition, multi-dimensional TIF image file format.
Flexible subarea Clip Scanning mode with all available image pixel formats to efficiently reduce the amount of acquired data and speed up frame rate.
Resonant Scanning
512x512 pixels, 12 bit per channel acquisition.
Rectangle clip scanning mode to efficiently reduce the amount of acquired data and speed up frame rate.
Multi-area Time Lapse (MATL) functionality
- XY stage control software module to perform advanced experiment procedures, implemented in FluoView application software.
- time-lapse experiments at a variety of points scattered over the sample are possible
- improved experiment efficiency in long duration experiments, as several points in the sample can be monitored in one experiment
- thousands of measuring points (differing in X, Y and Z) is possible
- as measurement conditions can be changed (points added/deleted etc.) during the experiment, flexibility is ensured
- Simultaneous up to 4 non-descanned fluorescence, multichannel fluorescence image with offered FVMPE-RS system configuration.
- User specific acquisition parameters and settings can be saved and recalled to repeat acquisition at same conditions.
TruResolution Automatic Correction Collar software control
- Software control for automatic correction collar
- System can be driven by an algorithm that finds the best collar settings for each depth
- Dynamic adjustment of correction collar for ideal settings throughout the volume
- Manual or automatic presets for three dimensional acquisitions
FluoView Application Software – post-acquisition display and analysis:
Implemented display and analysis functions:
- Possibility to save images in the following formats:Olympus Raw Image format (*.oir)
- Olympus Multi Tiff format (*.oib / *.oif)
- 8 / 16 bit grey scale / index colour, 24 / 32 / 38 bit colour images
- several image formats (*.jpg / *.bmp / *.tiff)
- movie format (*.avi)
- Zoom: 1x to 50x in 0.1x steps (1x to 8x with resonant scan)
- Multi-colour, multi-channel and multi-dimensional display, 1D to 4D (XYZ and t)
- Standard set of image processing filters and scalar processing (edge, low-pass, Sobel, Median, 2D Laplacian, edge enhancement), background shading correction for transmitted light images / DIC
- Image cropping in XYZT
- Measurements: histograms, time lapse tracing on lines, areas, ROI, on-line ROI display, etc.
- 3D volume rendering and time series animation
- TIME Course functionality for time course analysis and physiology experiment evaluation (incl. Ratio measurements), external trigger IN / OUT via FVMPE-RS control unit for synchronisation with external devices (e.g. electrophysiological instruments), online and post acquisition multiple ROI analysis.
- Quantitative colocalisation display and analysis
- Spectral deconvolution
Batch Image Analysis to set up several different image processing steps in a row
Additional analysis functions supplied by CellSens Dimension Desktop (also possible using Imaging PC1, which also has IMARIS software):
Along with threshold based phase analysis and offline kinetic functions, cellSens Dimension adds a range of image processing and analysis functions, for example arithmetic and logical operations, edge detection, projections calculations, image smoothing. Constrained Iterative Deconvolution
Tissue clearing
Deep imaging in tissue is challenging because of refractive index mismatch from immersion media and especially within the sample itself. In order to circumvent that issue in deep imaging tissue can be cleared. Tissue clearing can be quite complex, we are not experts yet, but below you will find information we find helpful:
Paper: Chemical Landscape for Tissue Clearing Based on Hydrophilic Reagents
FAQ
Animal Facility
- How do I get access to the Skou animal facility?
- In order to access the Skou animal facility, you will need to book a training session with the stable personnel first. Please directly contact the stable personnel for this matter.
- Which samples may I bring to the stables?
- You are NOT allowed to bring animals from other facilities to the Skou animal facility.
- Please contact the stable personnel for information on which and how to bring samples to the Skou animal facility.
Equipment
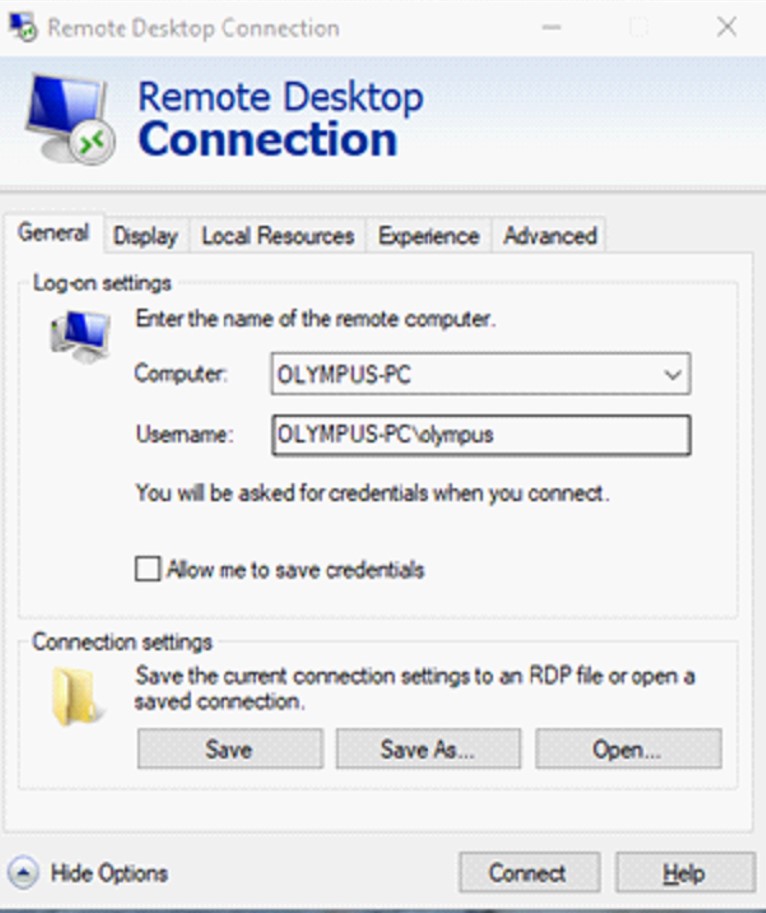
- Can I do 2-Photon imaging remotely?
2-p imaging, and access to the animal facility, can be time consuming. We do what we can to make it convenient for our users. After mounting a sample and starting up a long-time experiment, users can access the system via Remote Desktop. All imaging parameters and movements (x,y,z) can be controlled from your office PC (you need LAN cable, not Wifi). See image below for logon information and use the password that you will get from us personally. For detailed guide to remote desktop please follow this link. Shutting off the system requires your presence.


Need help?
Come to our Open Office sessions to discuss your imaging needs or get advice on workflows, free of charge:
Tuesdays and Thursdays 13:30 -14:30
The Skou building, 1116-256
Murine Lymph Nodes
by Thomas Rea Wittenborn, Ph.D., postdoc, Degn Lab, Department of Biomedicine, Aarhus University, Denmark
Blue: Collagen in lymph node capsules (second harmonics)
Red: Subcapsular macrophages
Green: Conduits through lymph nodes rapidly transporting antigen to the lymphocytes.