Spinning Disk Confocal, Olympus
Would you like your image to be displayed here?
Please contact us or send an email to imaging@biomed.au.dk, if you would like to share your images - Thank you
Applications in brief
The IX83 inverted spinning disk confocal microscope allows fast time-lapse confocal imaging of living cells with minimal phototoxicity and less photobleaching. Stability during long-term and high-speed imaging facilitates quantitative analysis of huge amounts of data.
Features
- Environment control (cellVivo CO2 and temperature control)
- Z-drift compensation system
Which samples may I use?
Selected samples, but not limited to:
- fixed cells
- fixed tissue
Training and use
Please familiarize yourself with the above guide containing info on:
- how to calibrate Z-limit for different stage inserts
- how to leave the system for the next user
Specifications
| Full equipment name | IX83 inverted spinning disk confocal microscope, Olympus |
| Laser | 4 line laser engine: iChroms MLE 405nm, 488nm, 561nm, 640nm |
| Camera | Hamamatsu ORCA-FusionBT sCMOS (formerly: Orca Fusion BT C15440) |
| Environment control | cellVivo CO2 and temperature control |
Objectives
| Mag./NA | Medium |
| 40x | Oil |
| 60x | Water |
| 100x | Oil |
| 100x | Silicon oil |
Fluorescence filters
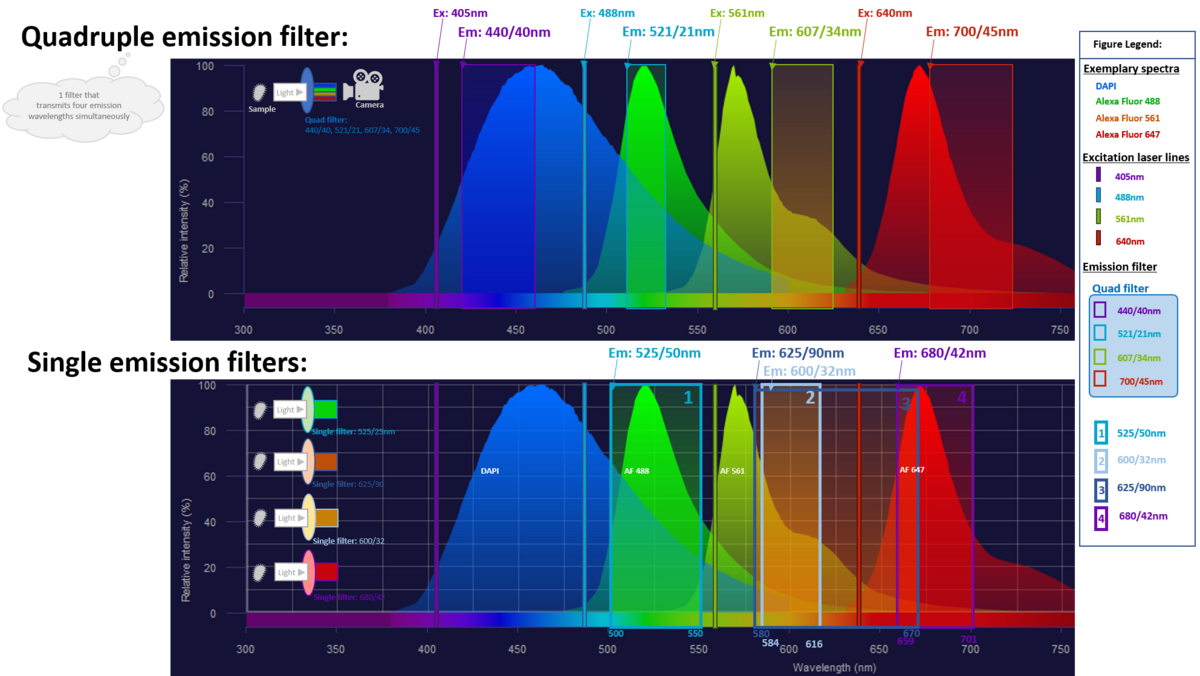
| Band pass filters (Confocal imaging) | Quad band filter: 440-40 / 521-21 / 607-34 / 700-45 |
| Band pass filter: 525-50 | |
| Band pass filter: 600-32 | |
| Band pass filter: 625-90 | |
| Band pass filter: 680-42 | |
| Filter cubes (Widefield ocular observation) | Triple band: Dapi/FITC/TRITC |
| Blue single band: Exciter filter: 360-370 nm, beam splitter: 410 nm, barrier filter: 420-460 nm | |
| Green single band: Exciter filter: 470-495 nm, beam splitter: 505 nm, barrier filter: 510-550 nm | |
| Red single band: Exciter filter: 565-585 nm, beam splitter 595, barrier filter: 600-690 |
Explainer: Fluorescence filters